Understanding Tumor Mutational Burden (TMB)
Most of us are familiar with the general concepts underlying the development of cancer: as our cells divide they acquire mutations that are generally harmless, until they’re not: with enough mutations in the wrong places, cells acquire the ability to divide much faster than normal. The newly cancerous cell divides uncontrollably, eventually developing into a tumor. And like a copy machine gone haywire, cancer cells have higher-than-average rates of mutation, leading to the buildup of a quantifiable number of mutations in the resulting tumor.
This number, called the tumor mutational burden (TMB), can be measured clinically via tumor tissue sequencing and is reported as mutations per megabase (mut/Mb) of DNA. Patients can be stratified into TMB-low and TMB-high buckets based on their sequencing results, with a score of 10 mut/Mb or higher defining high-TMB status. TMB is now known to have a significant impact on how individuals respond to different cancer treatments, with the bulk of the research done in the context of immunotherapy, a form of treatment where the body’s own immune system is activated against the tumor.
Normal cells become cancerous through the acquisition of mutations and subsequent uncontrolled division. Image created with Biorender.com
The first immunotherapy treatment was FDA-approved in 2011, and as early as 2014 scientists noted that patients whose cancers were classified as TMB-high were also more likely to respond to treatment. The most widely accepted hypothesis about why this might be suggests that the more mutations a cancer cell has, the less it looks like a normal cell and the more it resembles a foreign pathogen, the type of thing the immune system evolved to fight off.
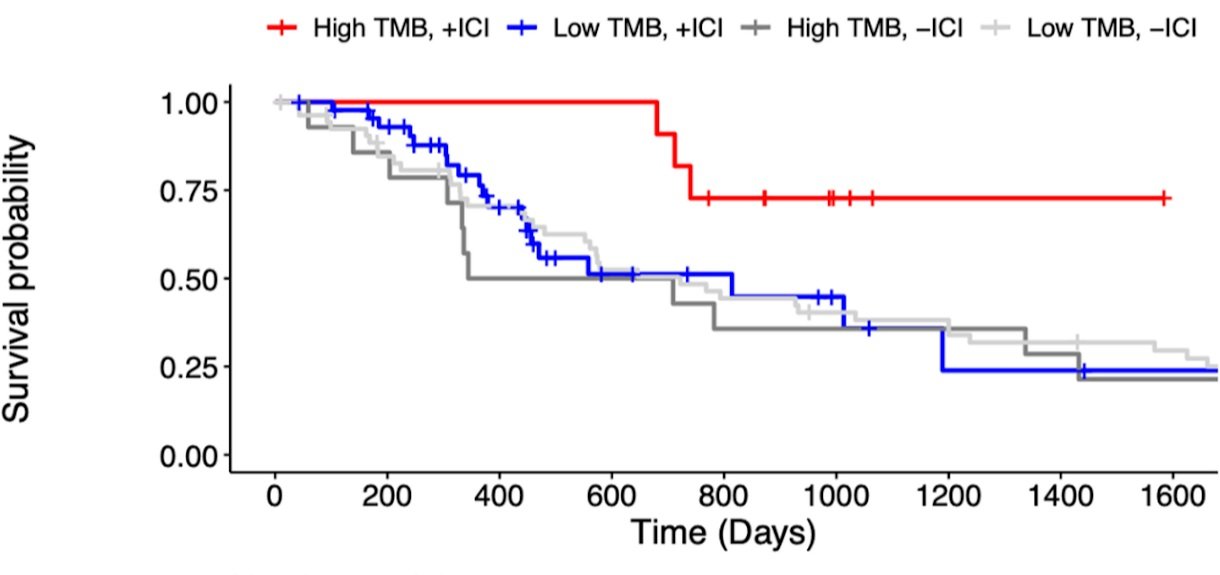
Kaplan-Meier overall survival curves for Stage IV renal cell carcinoma patients, stratified by immunotherapy treatment (+/- ICI) and tumor mutational burden (Low/High TMB). Patients with high TMB had higher overall survival when treated with immunotherapy than low-TMB patients. Data from Wood et al. Genome Med, https://doi.org/10.1186/s13073-020-00729-2
Although currently only a modest percentage of patients treated with immunotherapy (somewhere between 10-50% depending on the type of cancer and stage) will show positive responses in the form of tumor growth inhibition, that minority might experience some pretty unprecedented extended survival and long-term remission. Consequently, a major goal in cancer research right now is to understand and predict which types of tumors are likely to be affected by immunotherapy treatment. A test that could accurately predict whether a patient will respond to a given immunotherapy would be hugely beneficial, and TMB has emerged as a key biomarker in this effort.
Traditional TMB testing involves taking a biopsy of the tumor and sequencing the DNA that is extracted, but some patients don’t have enough tumor tissue, or cannot safely undergo the procedure. Liquid biopsies, which test a patient’s blood, are a game-changer in the goal of making TMB testing and its benefits available to all patients. Liquid biopsies are possible because as tumors develop and old cancer cells die, DNA is shed into the bloodstream. This circulating tumor DNA (ctDNA) can be sequenced just like a tumor biopsy to obtain mutational burden information, which is called blood TMB (bTMB). Although there is no FDA-approved bTMB test to date, several companies, including BLOODPAC members Guardant Health, Foundation Medicine, Tempus, and Personal Genome Diagnostics, have developed assays that assess a patient’s bTMB status and other genetic characteristics.
In general, a liquid biopsy involves taking a blood draw from a patient and analyzing the circulating cell-free DNA for the presence of cancer, such as tumor-derived DNA containing mutations or epigenetic modifications. Image created with BioRender.com
As with any new medical advance, safety and proof of efficacy are essential. With so many different players in this space, including clinical and translational laboratories, major research hospitals, and biotech companies, coming together to agree on how to use bTMB as a biomarker and how to validate new tests as they are developed against a common standard is a critical need. That’s where BLOODPAC comes in—the nonprofit was started in 2016 to accelerate the development, validation and clinical use of liquid biopsy assays to better inform medical decisions and improve patient care and outcomes. Today, BLOODPAC has over 60 members from regulatory, industry, and academic institutions, all of whom share data and experience to standardize the definition and analytic validation requirements for different types of liquid biopsy tests including bTMB.
As progress in developing and refining bTMB testing marches on, the scientific community is grappling with how best to use this biomarker, and how to combine it with other biomarkers to obtain the best possible predictive value. BLOODPAC is actively working to establish consensus on these questions, and developing frameworks for the scientific studies that will help answer them.