Understanding Liquid Biopsy for Early Cancer Detection and Screening
Advances in the prevention and early detection of cancer have historically lagged behind the development of new cancer treatments. In the past decade, we’ve seen dozens of new targeted molecular therapies and immunotherapies introduced. However, when it comes to screening for cancer, the tests we use today are largely unchanged from those our parents used: mammograms, Pap smears, and colonoscopies. Tests currently recommended by the US Preventative Services Task Force only cover four cancers–breast, cervical, colorectal, and lung–representing less than half of all US cancer cases. We have no screening tests that are recommended for asymptomatic individuals for most cancers, including some of the most lethal cancers, and low adoption of these screening guidelines means that only 14% of cancers are detected through a preventative screen (1). Many individuals with cancer are diagnosed in later stages after symptoms appear, significantly reducing the chance of survival.
Liquid biopsies are poised to change this. Advances in blood-based cancer detection in the past several years have led to numerous high-profile studies, several tests on or entering the market, and hundreds of thousands of individuals enrolled in clinical trials.
In this article, we’ll cover:
How liquid biopsies for cancer early detection and screening (EDS) work
EDS tests in development
The potential impact of EDS tests on population-wide health
Remaining challenges and open questions in the field
How do liquid biopsies for early cancer detection & screening work?
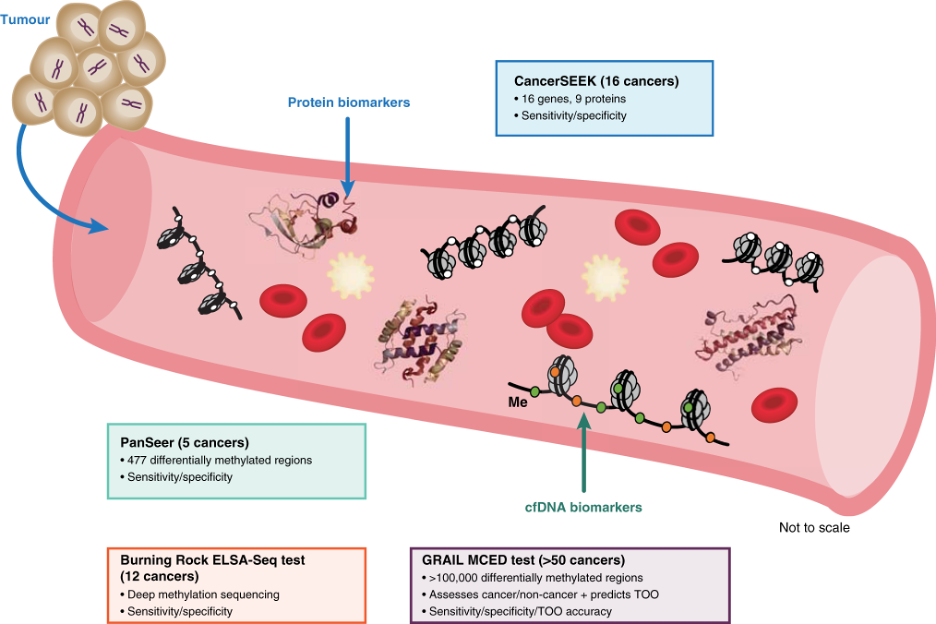
Practically, liquid biopsy testing involves taking a blood (or other biological fluid) sample from a patient, isolating circulating free DNA (cfDNA) or other biomolecules, and subjecting the sample to sequencing, PCR, or other detection methods to identify cancer-derived signals. Some liquid biopsy tests are already FDA-approved as companion diagnostics for therapy selection in multiple cancer types, while others are being used to monitor for cancer recurrence and treatment response in late-stage disease (see here for more on this topic). The current generation of EDS tests are largely based on cfDNA.
As a method for detecting cancer before clinical signs or symptoms appear, liquid biopsy tests must be highly sensitive because the amount of cancerous DNA in circulation is typically much lower than in late-stage cancer (2,3). In addition, some cancers, especially more aggressive and better vascularized types, tend to release DNA into the bloodstream more readily than others (2). A stage I cancer will typically release less DNA than a stage II cancer even though both may be asymptomatic. This differential DNA shedding means that cfDNA-based assays can have a relatively wide range of sensitivity depending on which cancer and stage is being assessed. At the same time, EDS tests must be very specific to cancerous signals to avoid false positives, which can subject patients to unnecessary invasive testing. To achieve high specificity and sensitivity, EDS tests can be “multi-modal” and/or “multi-omic,” meaning that cancer-derived cfDNA mutations, cfDNA methylation and/or other cfDNA-based markers, may be combined with other biomolecules, such as cancer-associated proteins, to determine the result (4).
Blood-based EDS tests to detect both single cancers and multiple cancers are currently in late-stage development. One feature built into some multi-cancer detection (MCD) tests is the ability to predict the source(s) of the cancer signal in the event of a positive result, called the tissue or site of origin.
What cancer early detection and screening tests are being developed?
Several liquid biopsy early detection assays are currently in late-stage development or clinical trials. One single-cancer detection test, Epigenomics’ ProColon, is FDA-approved for detection of colorectal cancer but was discontinued by the manufacturer this year. The ProColon test detects colorectal cancer-specific methylation patterns in cfDNA and was indicated for individuals eligible for colorectal cancer screening who have a history of not completing the recommended screenings.
An overview of several multi-cancer detection assays in development and commercially available. Figure from Liu (2021), British Journal of Cancer.
GRAIL’s Galleri test, which utilizes cancer-specific methylation patterns in cfDNA to detect more than 50 cancer types, is a MCD assay available as a laboratory-developed test (5,6). GRAIL reports 99.5% specificity, suggesting a false positive rate of approximately 1 in 200 (5,7,8). In the most recently published results of GRAIL’s SYMPLIFY trial, an evaluation of the Galleri test in over 6,000 individuals who were referred for diagnostic follow-up on suspicion of cancer, the test’s sensitivity ranged from 24% for stage I cancer to 99% in stage IV (9). When a positive signal was detected among patients with cancer, the test's prediction of the tissue of origin was accurate in 85% of cases (9).
GRAIL has partnered with the National Health System (NHS) in the UK to evaluate the clinical utility of the Galleri assay. In this randomized controlled trial, 165,000 asymptomatic individuals between the ages of 50 and 79 will have a blood test each year for three years to check for the presence of malignant tumors (10). Other ongoing studies of the Galleri test include the prospective STRIVE (NCT03085888) and SUMMIT (NCT03934866) trials in intended-use populations, and the prospective, interventional PATHFINDER study (NCT04241796). In the PATHFINDER trial, test results are returned to clinicians allowing for follow-up testing if cancer is detected.
Exact Sciences is also developing an assay based on CancerSEEK technology, an EDS test that uses multiple biomarkers including proteins and DNA to detect eight to ten cancer types (11). This test has a breakthrough device designation from the FDA for the detection of ovarian and pancreatic cancer. The precursor of the Exact assay was used in the DETECT-A study, which combined two rounds of blood testing with PET imaging to detect the presence of cancer (12). Another EDS test is in development at DELFI Diagnostics. The DELFI assay is based on fragmentation patterns of cfDNA, which have been shown to be distinct in individuals with cancer as compared to healthy controls (13).
In the single-cancer early detection space, Guardant is developing the Guardant Shield, a single-cancer liquid biopsy detection assay for colorectal cancer. The Shield tests combines methylation patterns and analysis of DNA fragment genomic position (fragmentomics) to predict colorectal cancer with 90% specificity and 83% sensitivity (14,15). This assay is currently being evaluated in the ECLIPSE study (NCT04136002), with over 20,000 average-risk patients enrolled. The Shield test is also available as a laboratory-developed test.
Several other early detection tests in development include Burning Rock’s OverC test, an MCD assay which received breakthrough designation in January 2023, and Singlera Genomic’s PanSeer, which detects cancer-specific DNA methylations patterns.
What is the potential impact of blood-based early detection and screening tests on population health?
Several groups have published on the potential reductions in cancer mortality and other metrics following widespread implementation of a blood-based MCD test, but broad uncertainty remains on what impact this technology will have in the near future. One recent modeling study evaluating the clinical and economic implications of an assay detecting 19 major cancers in asymptomatic US adults as compared to existing single-cancer screening estimated a 53% reduction in stage IV cancer diagnoses, longer overall survival, and a decrease in per-cancer treatment costs. Another study, based on performance data from GRAIL’s Galleri test, reported a 78% reduction in late stage (III and IV) cancer among 55-79 year-olds, leading to a 26% overall reduction in all cancer-related deaths (17). In a study modeling the impact of population-wide adoption of the GRAIL Galleri test among 50-79 year-old in the UK, authors reported a reduction of at least 17% in cancer deaths per year under least-favorable model conditions (18).
All of these studies make several assumptions as part of the modeling process, including near-universal adoption of a given MCD assay. Of course, real-world conditions are far from perfect, and the ultimate impacts of such a screening program will be heavily influenced by implementation. A recent commentary on the impact of MCD testing emphasized the critical gaps in our knowledge that make it effectively impossible to reliably predict overall population benefit (19). The authors identify the lack of guidance for confirming positive MCD test results and finding the tissue of origin, the population testing strategies and guidelines created, and the lack of medical knowledge about the natural history of cancers that currently have no screening tests as major variables.
What challenges and open questions remain in blood-based cancer screening?
Blood-based EDS tests, and particularly multi-cancer detection tests, are novel enough that many unknowns remain along the journey to widespread real-world use. A major challenge in the field is determining how the clinical validity—testing which ensures the assay functions as intended—should be evaluated. If an assay detects 50 different cancers, does each the assay’s ability to detect each one need to be tested separately? How should the limit of detection for this assay be determined? If MCD tests were to be clinically validated using the blueprint of single-cancer screening tests, such a study would need to enroll enough individuals to find a statistically relevant number of every type of cancer tested.
Another evolving discussion revolves around clinical utility: demonstrating an improvement in health outcomes resulting from use of an EDS test. Individual rates of cancer incidence are generally very low, and EDS tests purport to detect cancer earlier than is currently possible. Studies assessing clinical utility would therefore need to follow individuals for years to observe the effect of the test on mortality and demonstrate true benefit. Needless to say, a study of this type would be massive and extremely costly.
The EDS field is exploring new methods and study designs to address these points and answer critical questions such as:
Who should take blood-based cancer screening tests? What ages/demographics/populations receive the greatest benefit with smallest negative effects (minimizing unnecessary testing, follow-up screening, cost)?
Do blood-based cancer screening tests work equally well for all demographics and individuals?
How is the early cancer signal detected by a blood-based test different from a cancer diagnosis through conventional means?
What types of follow-up testing, monitoring, and treatment lead to the best outcomes?
How do we evaluate the social and economic value of blood-based cancer testing?
How do we implement these assays to ensure blood-based cancer testing doesn’t exacerbate the existing health disparities in cancer outcomes?
Finding thoughtful and innovative solutions to these issues is beyond any single stakeholder in the EDS liquid biopsy space. Collaborative nonprofits such as the Friends of Cancer Research, the MCED Consortium, and BLOODPAC are actively working to address these challenges by bringing together patient advocacy, nonprofit, governmental/regulatory, industry and academic organizations working in liquid biopsy for cancer early detection.
BLOODPAC’s Early Detection and Screening working group is comprised of subject-matter experts from assay developers, not-for-profits, academic institutions, and pharmaceutical industries including GRAIL, Natera, Exact Sciences, Guardant, the National Cancer Institute, USC, DELFI Diagnostics, Prevent Cancer Foundation, Friends of Cancer Research, AstraZeneca, Novartis, Foundation Medicine, Bristol Meyers Squibb, and Glaxo Smith Klein.
The working group published an overview of challenges and opportunities in this space in the January 2023 issue of Clinical & Translational Science, and is now finalizing an EDS lexicon, a compendium of agreed-upon definitions for key terms. The lexicon is intended to help ensure clear and consistent communication among stakeholders who use, assess, or interpret EDS tests including clinicians, regulatory agencies, payers, and patients.
BLOODPAC’s Accessibility working group is tackling another aspect of early cancer screening challenges: how to make sure that the rollout of these tests doesn’t deepen the existing inequities in precision medicine and cancer treatment. The group’s analysis of the barriers to accessing liquid biopsy-based EDS in the general population and within underserved communities will be published in the near future. Follow-up initiatives are planned to create the infrastructure and data to ensure that the next decade will bring equitable adoption of this novel precision medicine technology to every community in need.
Works Cited
1. New Research Highlights Just One In Seven Diagnosed Cancers Found By A Recommended Screening Test | NORC at the University of Chicago. https://www.norc.org/research/library/new-research-highlights-just-one-in-seven-diagnosed-cancers-foun.html.
2. Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6, (2014).
3. Crosby, D. Delivering on the promise of early detection with liquid biopsies. British Journal of Cancer Preprint at https://doi.org/10.1038/s41416-021-01646-w (2022).
4. Keller, L., Belloum, Y., Wikman, H. & Pantel, K. Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. British Journal of Cancer 2020 124:2 124, 345–358 (2020).
5. Clinical Evidence | Galleri® for HCPs. https://www.galleri.com/hcp/clinical-evidence.
6. Liu, M. C. et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Annals of Oncology 31, 745–759 (2020).
7. Alexander, G. E. et al. Analytical validation of a multi-cancer early detection test with cancer signal origin using a cell-free DNA–based targeted methylation assay. PLoS One 18, e0283001 (2023).
8. Oxnard, G. R. et al. Simultaneous multi-cancer detection and tissue of origin (TOO) localization using targeted bisulfite sequencing of plasma cell-free DNA (cfDNA). JCO Glob Oncol 5, 44–44 (2019).
9. Nicholson, B. D. et al. Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): a large-scale, observational cohort study. The Lancet (2023) doi:10.1016/S1470-2045(23)00277-2.
10. Neal, R. D. et al. Cell-Free DNA–Based Multi-Cancer Early Detection Test in an Asymptomatic Screening Population (NHS-Galleri): Design of a Pragmatic, Prospective Randomised Controlled Trial. Cancers (Basel) 14, 4818 (2022).
11. Exact Sciences. Information for providers | Multi-cancer early detection. https://www.exactsciences.com/Pipeline-and-Data/multi-cancer-early-detection/providers.
12. Lennon, A. M. et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science (1979) 369, (2020).
13. Cristiano, S. et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 570, 385–389 (2019).
14. Guardant Health. Guardant Health announces positive results from pivotal ECLIPSE study evaluating a blood test for the detection of colorectal cancer. https://investors.guardanthealth.com/press-releases/press-releases/2022/Guardant-Health-announces-positive-results-from-pivotal-ECLIPSE-study-evaluating-a-blood-test-for-the-detection-of-colorectal-cancer/default.aspx.
15. Kim, S.-T. et al. Abstract 916: Combined genomic and epigenomic assessment of cell-free circulating tumor DNA (ctDNA) improves assay sensitivity in early-stage colorectal cancer (CRC). Cancer Res 79, 916–916 (2019).
16. Tafazzoli, A. et al. The Potential Value-Based Price of a Multi-Cancer Early Detection Genomic Blood Test to Complement Current Single Cancer Screening in the USA. Pharmacoeconomics 40, 1107–1117 (2022).
17. Hubbell, E., Clarke, C. A., Aravanis, A. M. & Berg, C. D. Modeled reductions in late-stage cancer with a multi-cancer early detection test. Cancer Epidemiology Biomarkers and Prevention 30, 460–468 (2021).
18. Sasieni, P. et al. Modelled mortality benefits of multi-cancer early detection screening in England. British Journal of Cancer 2023 1–9 (2023) doi:10.1038/s41416-023-02243-9.
19. Etzioni, R., Gulati, R. & Weiss, N. S. Multicancer Early Detection: Learning From the Past to Meet the Future. JNCI Journal of the National Cancer Institute 114, 349 (2022).